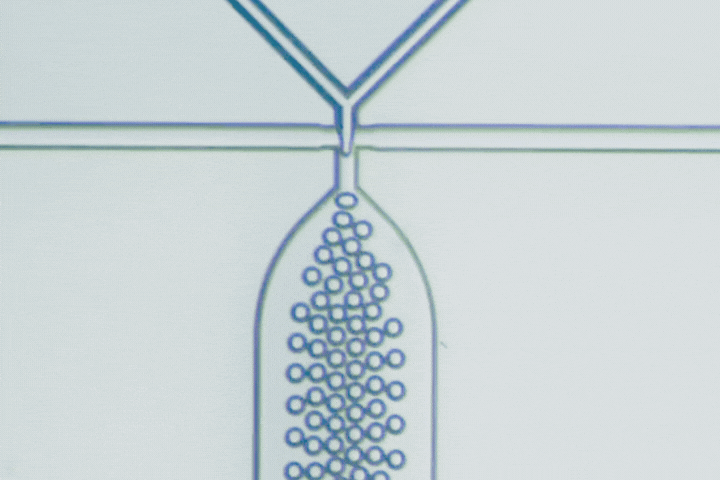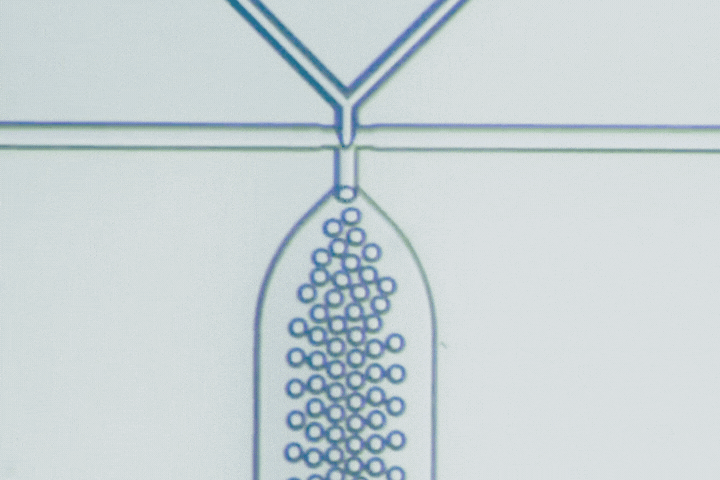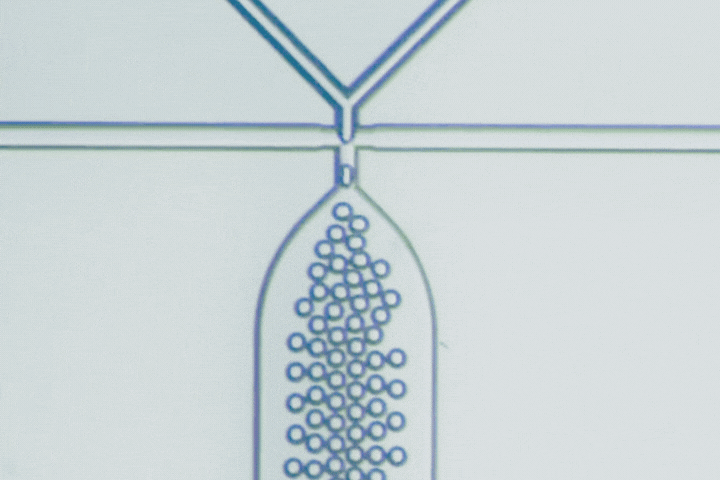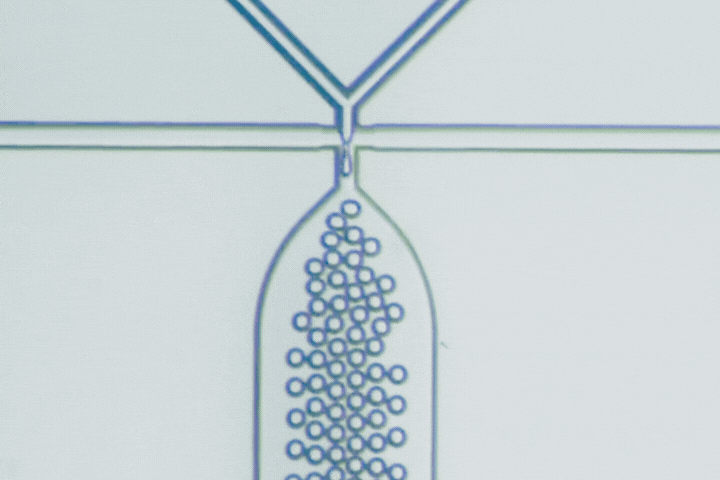
Beyond PLGA: Exploring new materials for microfluidic drug delivery
PLGA has long set the standard for controlled drug delivery, but modern therapeutics like mRNA, CRISPR, and personalised oncology expose its limits. This review explores emerging materials beyond PLGA - from lipid nanoparticles and smart polymers to microgels and biomimetic systems - and shows how microfluidics enables precise, scalable fabrication for next-generation drug delivery applications.
Poly (lactic-co-glycolic acid) (PLGA) has been a cornerstone in controlled release drug delivery, enabling the translation of therapies from the lab to the clinic due to its proven biodegradability and biocompatibility. However, the emergence of modern therapeutics, such as mRNA vaccines, CRISPR gene-editing, and personalized oncology agents, has revealed PLGA's limitations. These include poor encapsulation of hydrophilic or nucleic acid payloads, prominent burst release, rapid immune clearance, and insufficient adaptability to disease microenvironments.
Microfluidics has emerged as a key technology to overcome these challenges, using microscale channels for rapid, uniform mixing and nanoprecipitation, producing particles with narrow size distributions (polydispersity index - PDI below 0.2). This technology can achieve high encapsulation efficiencies (85-95%) even for delicate biologics, as well as complex particle structures like core-shell, multilayer, and Janus particles that conventional bulk methods can’t replicate.
Today, there is a growing focus on sustainable, bio-derived materials integrated with microfluidic platforms, driven by regulatory emphasis on eco-friendly manufacturing and the need for solvent-minimized processes.
This review explores innovative materials beyond PLGA, including LNPs and biomimetic hybrids, focusing on their microfluidic-enhanced performance and real-world applications in drug delivery.
1. Lipid nanoparticles: Powering the era of genetic medicines
Lipid nanoparticles have redefined delivery for nucleic acids (e.g., mRNA, siRNA, pDNA…), offering excellent biocompatibility, tuneable biodegradation, and the ability to disrupt endosomal membranes via ionizable lipids. Their ascent, propelled by mRNA vaccines, extends to siRNA for gene silencing and CRISPR for gene editing where PLGA falters due to poor nucleic acid compatibility.

Microfluidics excels in this area by using techniques like hydrodynamic flow focusing and staggered herringbone mixers to achieve rapid nanoprecipitation, producing particles with sizes of 20–100 nm and encapsulation efficiencies up to 95%. This precision optimises biodistribution, with smaller LNPs penetrating tumours via EPR, while larger ones facilitate lymphatic drainage for vaccines [1].

2. Smart polymers for tuneable and targeted release
Stimuli-responsive polymers surpass PLGA by adapting to disease microenvironments, swelling at low pH for tumour release or degrading upon exposure to local enzymes. Examples include PEGylated block copolymers for stealth circulation, poly(β-amino esters) (PBAEs) that enable electrostatic loading of nucleic acids, and polycaprolactone (PCL) for ultra-slow degradation over months.
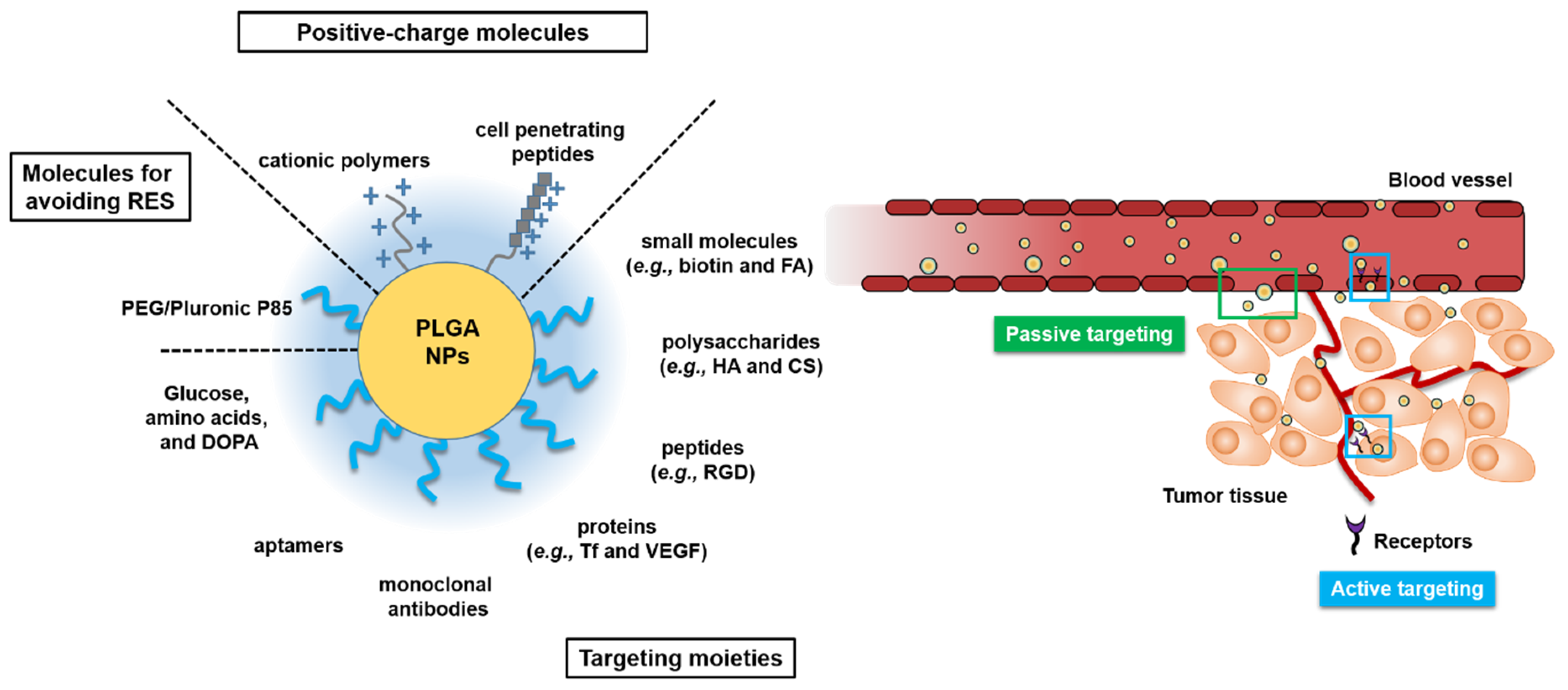
Although PLGA remains the clinical benchmark, exemplified by Lupron Depot®, where leuprolide acetate encapsulated in PLGA microparticles achieves long-acting, controlled release, innovation is moving beyond traditional PLGA. Hybrid systems, such as thermosensitive PLGA–PEG–PLGA triblock copolymers, form in situ gelling depots. These formulations can sustain the release of glucagon-like peptide-1 (GLP-1) agonists for weeks, cutting injection frequency by up to 75% in diabetes therapy.
Microfluidics further elevates these materials, enabling precise emulsification and the fabrication of core–shell or Janus particles that dramatically suppress PLGA’s initial burst (to <10%) while supporting multi-stage release. For example, T-junction droplet mixers can generate particles with distinct hydrophobic and hydrophilic compartments for more sophisticated release profiles.
3. Microgels and hybrid hydrogels for injectable depots
Hydrogels mimic extracellular matrix with their softness and swelling, ideal for site-specific drug delivery in areas such as arthritis joints, post-surgical voids, ocular sites, and bone lesions. Their microgel variants add injectability and create microporous niches that promote cell spreading, nutrient transport, and early tissue ingrowth.
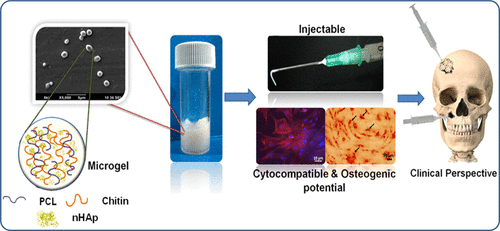
For example, chitin-PCL-nanohydroxyapatite (chitin-PCL-nHAp) microgels are highly injectable and support bone tissue engineering, aiding mesenchymal stem cell (MSC) differentiation into bone-forming cells. Similarly, gelatin-derived microporous hydrogel improves MSC osteogenesis and bone repair in vivo, showing a significant increase in alkaline phosphatase activity and calcium deposition.
Recent advances in microfluidics have expanded this area. Microfluidic alginate microgels crosslinked with Ca²⁺ promote osteogenic differentiation and matrix mineralization. Meanwhile, composite microspheres with sustained Mg²⁺ release enhance osteogenesis, angiogenesis, and bone regeneration in vivo [5]. These microfluidic platforms offer precise control over size, monodispersity, and compartmentalisation, enabling next-generation injectable depots with programmable biological responses.
4. Biomimetic designs: nature-inspired stealth and targeting
Biomimetic nanoparticle carriers, inspired by cellular exteriors, leverage natural membrane proteins to evade immune clearance and achieve precise targeted disease sites. Cell membrane-coated nanoparticles (CMCNPs) fuse synthetic cores (like PLGA or mesoporous silica) with membranes from red blood cells (RBCs), platelets, or cancer cells, incorporating "don't-eat-me" signals like CD47 to mimic host cells and extend their circulation time in the body.
A study by Gao et al. found that RBC-coated nanoparticles stayed in the blood for about 39.6 hours, compared to just 15.8 hours for PEGylated nanoparticles. This demonstrates how CD47 helps to evade the immune system [6]. In a mouse tumour model, doxorubicin (DOX)-loaded RBC-coated nanoparticles worked better at reducing tumour size and caused less overall toxicity than free DOX, proving the benefits of this immune-evading approach [7].
Microfluidic electroporation techniques improve these systems by creating uniform nanoscale vesicles (100–200 nm). This method ensures precise insertion of nanoparticle cores into the membrane fragments without damaging protein functionality, yielding reproducible coatings and scalable production suitable for translational nanomedicine.
Charting tomorrow's therapeutics
While PLGA laid the foundation for controlled release, modern therapeutics now demand a broader material toolkit. New delivery systems include LNPs for mRNA and gene editing, polymer–lipid hybrids for enhanced intracellular trafficking, and microgels or hydrogels for localised or cell-compatible release. Bio-derived matrices, such as alginate and hyaluronic acid derivative, offer biocompatibility and tuneable mechanics for regenerative and advanced therapies.
The future of drug delivery won’t be defined by one “best” material, but by how smart chemistry intersects with precise engineering to create delivery systems that are tailored, predictable, and clinically scalable.
Microfluidics is key to this evolution, enabling precise mixing and particle formation. It produces uniform nanoparticles, microgels, and hybrid constructs that are difficult to achieve by bulk methods. These capabilities accelerate the development of delivery systems that are smaller, smarter, and more clinically predictable.
At Blacksheep Sciences, we help partners explore these advanced technologies, using flexible and scalable microfluidic platforms to guide the development of effective and impactful therapies.
Explore how we can propel your pipeline toward efficient, impactful therapies: https://www.blacksheepsciences.com/
References:
[1]: Lipid Nanoparticles-From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 11, 16982–17015
[2]: Systemic delivery of blood–brain barrier-targeted polymeric nanoparticles enhances delivery to brain tissue. Journal of Drug Targeting, 23(7–8), 736–749
[3]: Poly(beta-amino ester)-Based Nanoparticles Enable Nonviral Delivery of Base Editors for Targeted Tumor Gene Editing. Biomacromolecules 2022, 23, 2116−2125
[4]: Polyanhydride Chemistry. Biomacromolecules 2022, 23, 12, 4959–4984
[5]: Microfluidic-templating alginate microgels crosslinked by different metal ions as engineered microenvironment to regulate stem cell behavior for osteogenesis. Materials Science & Engineering C 131 (2021) 112497
[6]: Preparation and Application of Cell Membrane-Camouflaged Nanoparticles for Cancer Therapy. Theranostics 2017; 7(10):2575-2592
[7]: Safe and Immunocompatible Nanocarriers Cloaked in RBC Membranes for Drug Delivery to Treat Solid Tumors. Theranostics 2016 Apr 28;6(7):1004-11.
.JPG)